Abstract
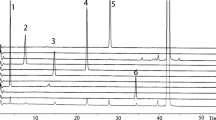
An HPLC method has been developed for the separation of valdecoxib and a degradation product consisting of α and β-N-lactosyl sulfonamide, i.e. α and β anomers (SC-77852). Best results were achieved with a Chromolith Performance RP-18e column (100 mm × 4.6 mm), macropore size 2 μm, mesopore size 13 nm, with an eluent of methanol:water containing a 1% solution of TEA (36:64 v/v), pH 7.4 (adjusted with 85% orthophosphoric acid), at 22 °C. Detection was at 220 nm. The method was validated for its selectivity, linearity, precision (repeatability) and robustness. Quantitation and detection limits were determined for both valdecoxib and SC-77852. Method robustness was further evaluated by performing 23 full factorial design experiments. The final step, optimisation of the variables, was performed using response surface design. The validated method was used for assay of valdecoxib and SC-77852 in Bextra® film-coated tablets.



Similar content being viewed by others
References
Abraham DJ (2003) Burger’s, medicinal chemistry and drug discovery. In: Autocoids, diagnostics and drug from new biology, 6th edn, vol 4. Wiley, New York
Zečević M, Savić G, Živanović Lj (2006) Anal Lett 39:1875–1890
Zhang JY, Douglas MF, Breau AP (2003) J Chromatogr B 785(1):123–134
Zhang JY, Douglas MF, Breau AP (2003) J Pharm Biomed Anal 33(1):61–72
Ramakrishna NVS, Vishwottam KN, Wishu S, Koteshwara M (2004) J Chromatogr B 802(2):271–275
Sane RT, Menon S, Deshpande AY, Jain A (2005) Chromatographia 61(3/4): 137–141
Werner U, Werner D, Hinz B, Lambrecht C, Brune K (2005) Biomed Chromatogr 19(2):113–118
Srinivas MS, Srinivas LD, Sastry BS (2004) Asian J Chem 16(2):1119–1123
Zarghi A, Shafaati A, Foroutan SM, Khoddam A (2006) J Chromatogr B 835(1/2):100–104
Vallano PT, Mazenko RS, Woolf EJ, Matuszewski BK (2002) J Chromatogr B 779(2):249–257
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (2003) ICH harmonized tripartite guideline, Topic Q3A(R)
Nederkassel AM van, Aerts A, Dierick A, Massart DL, Vander Heyden Y (2003) J Pharm Biomed Anal 32(2):233–249
Cabrera K, Lubda D, Eggenweiler H, Minakuchi H, Nakanishi K (2000) J High Resol Chromatogr 23(1):93–99
Ahuja S (1998) Impurities evaluation of pharmaceuticals. Marcel Dekker, NewYork
Ahuja S, Alsante KM (2003) Handbook of isolation and characterisation of impurities in pharmaceuticals. Elsevier, San Diego
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (2005) ICH Harmonised Tripartite Guideline Topic Q2(R1)
The United States Pharmacopeia 23rd revision, United States Pharmacopeial convention, Inc., 12601 Twinbrook Parkway, Rockville
Vander Heyden Y, Nijhuis A, Smeyers-Verbeke J, Vandeginste BGM, Massart DL (2001) J Pharm Biomed Anal 24(5/6):723–753
Fabre H (1996) J Pharm Biomed Anal 14(8/10):1125–1132
Lundstedt T, Seifert E, Abramo L, Thelin B, Nyström Å, Pettersen J, Bergman R (1998) Chemom Intell Lab Syst 42(1/2):3–40
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1365/s10337-007-0328-1
Rights and permissions
About this article
Cite this article
Savić, G., Zečević, M., Jocić, B. et al. Validation of an HPLC Method for the Determination of Valdecoxib and its Degradation Product: a Mixture of α- and β-n-Lactosyl Sulfonamide Anomers. Chroma 66, 29–35 (2007). https://doi.org/10.1365/s10337-007-0276-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-007-0276-9